Document Studio: Step-by-Step Guide
Overview
The Document Studio in INVISION allows users to set up, sign, and manage PDF documents efficiently. This guide will walk you through the three main parts of the Document Studio workflow:
- Admin Setup of the Document in Protocol
- User Signing in View Fields
- Emailing to Request Signature Signing
Part 1: Admin Setup in Protocol
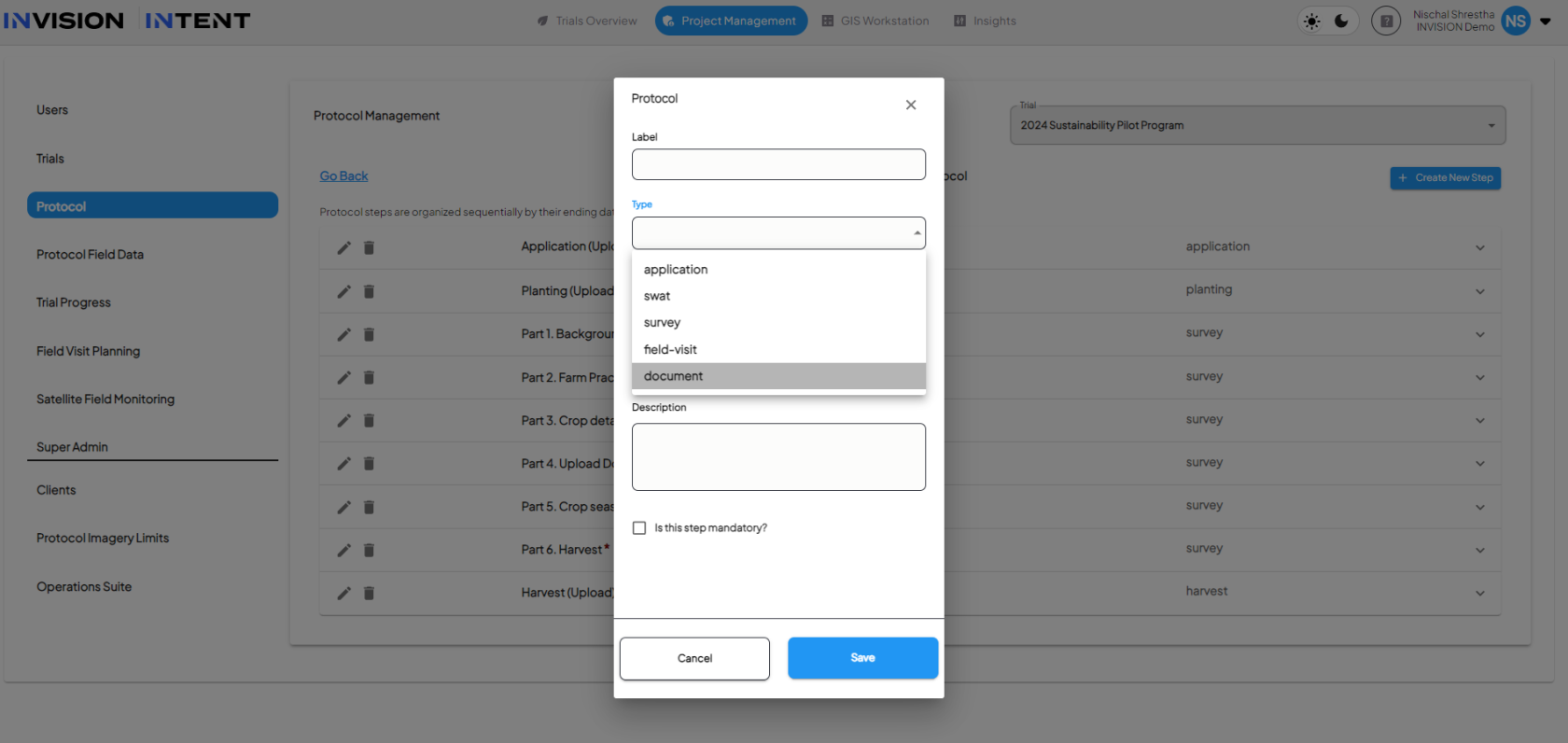
1. Create a New Protocol Step
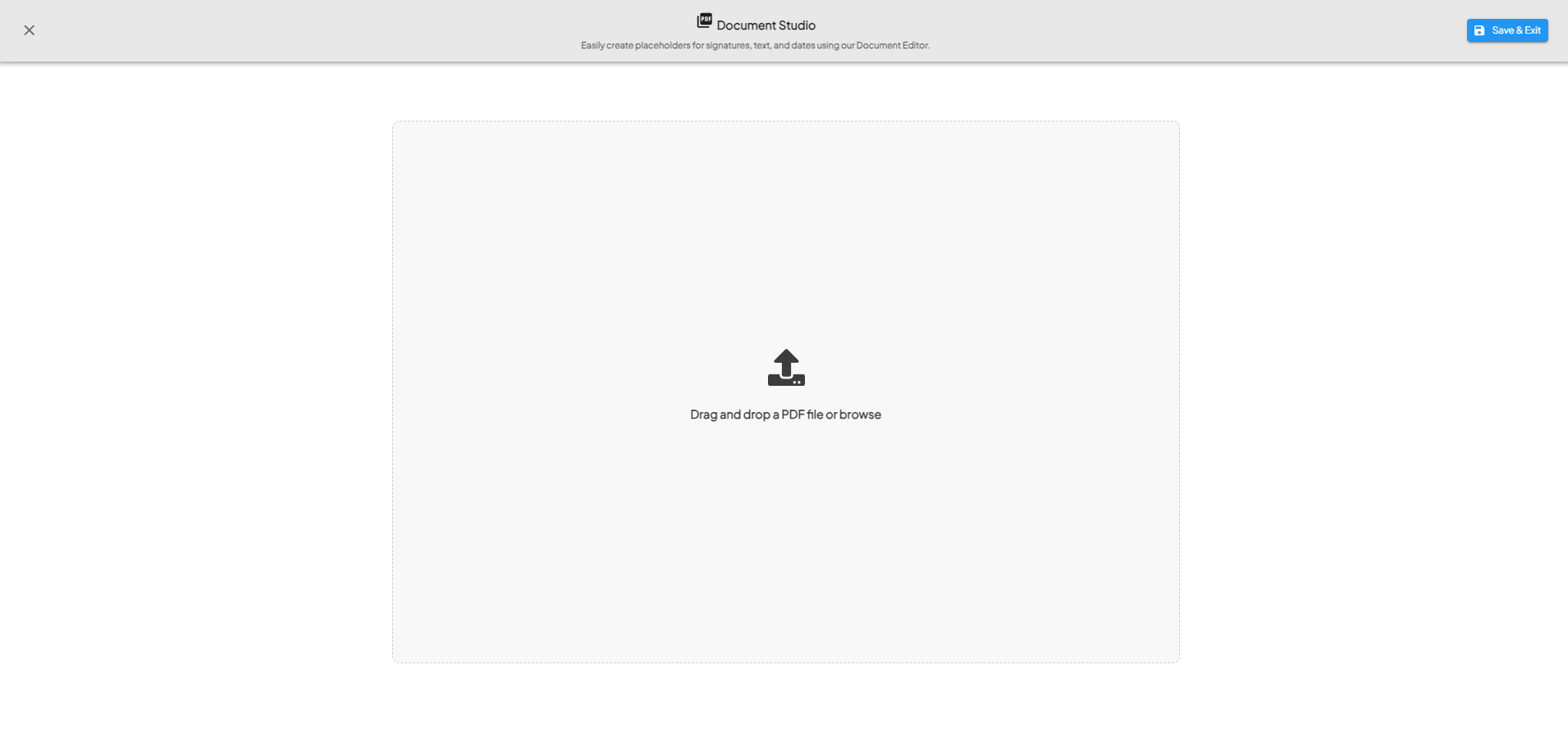
2. PDF Upload
- Use the drag-and-drop feature or click to browse to upload the PDF.
- You can only upload one PDF per step.
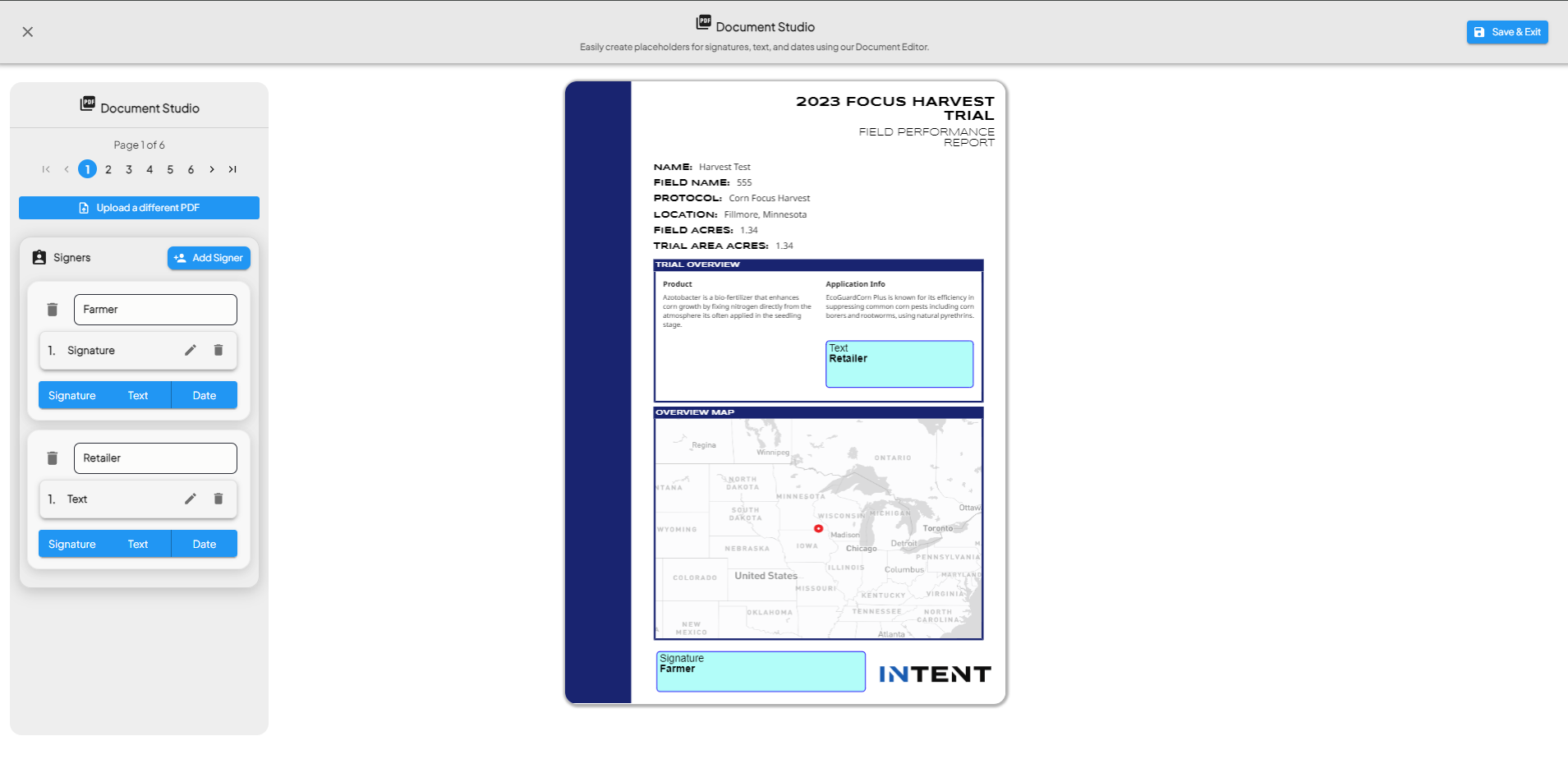
Annotate the PDF
- Use the PDF viewer to switch pages and scroll through the document.
- Add signers and create annotation steps using the buttons on the left panel for each signer:
- Click and Drag to create these following annotations:
- Add a signature
- Add text
- Add date stamps
- Reference to the drawing tool to place annotations.
- Annotations are displayed with labels in a sidebar.
- Once you are done creating annotations, hit Save & Exit.
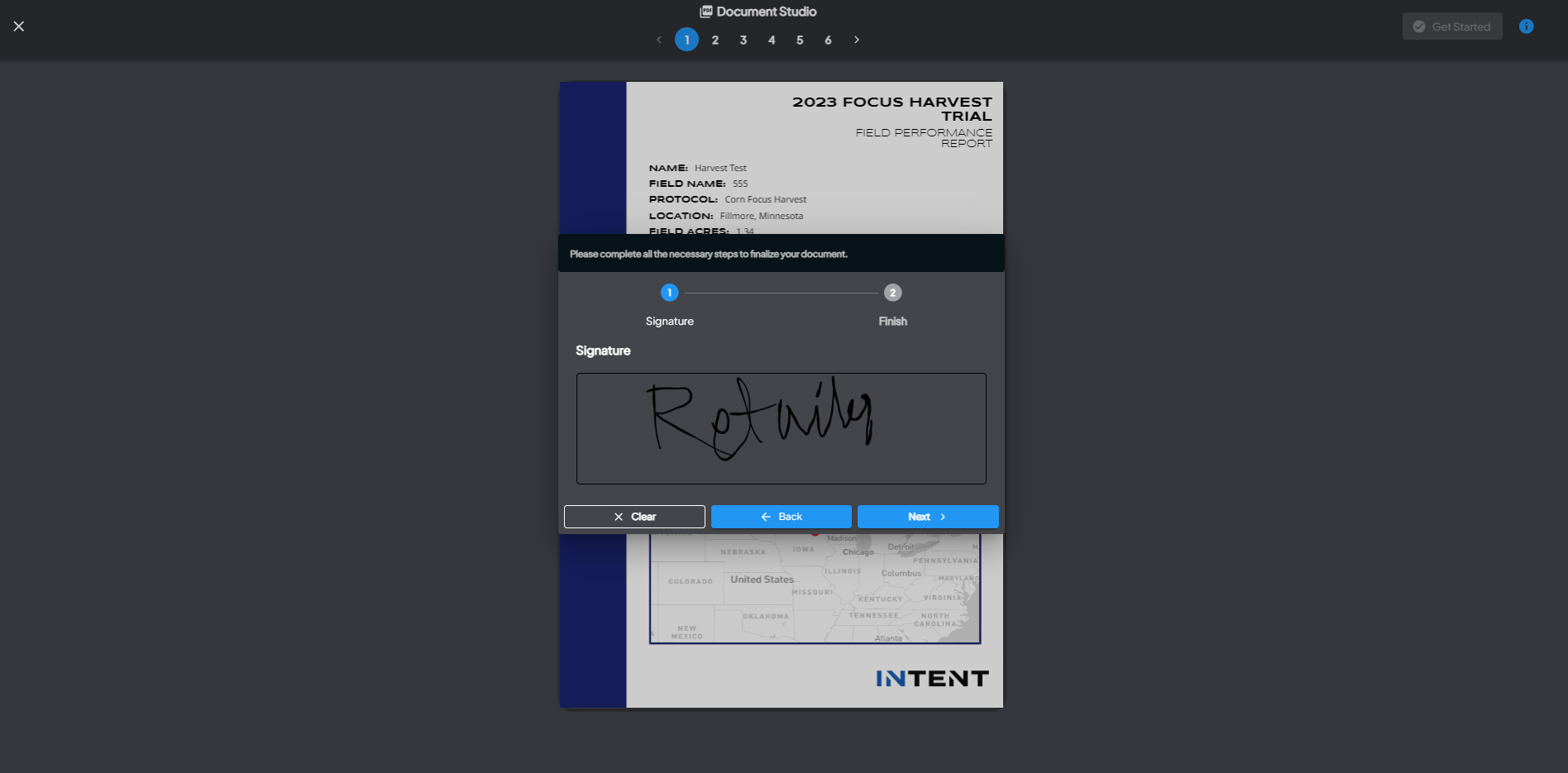
Part 2: User Signing in View Fields
-
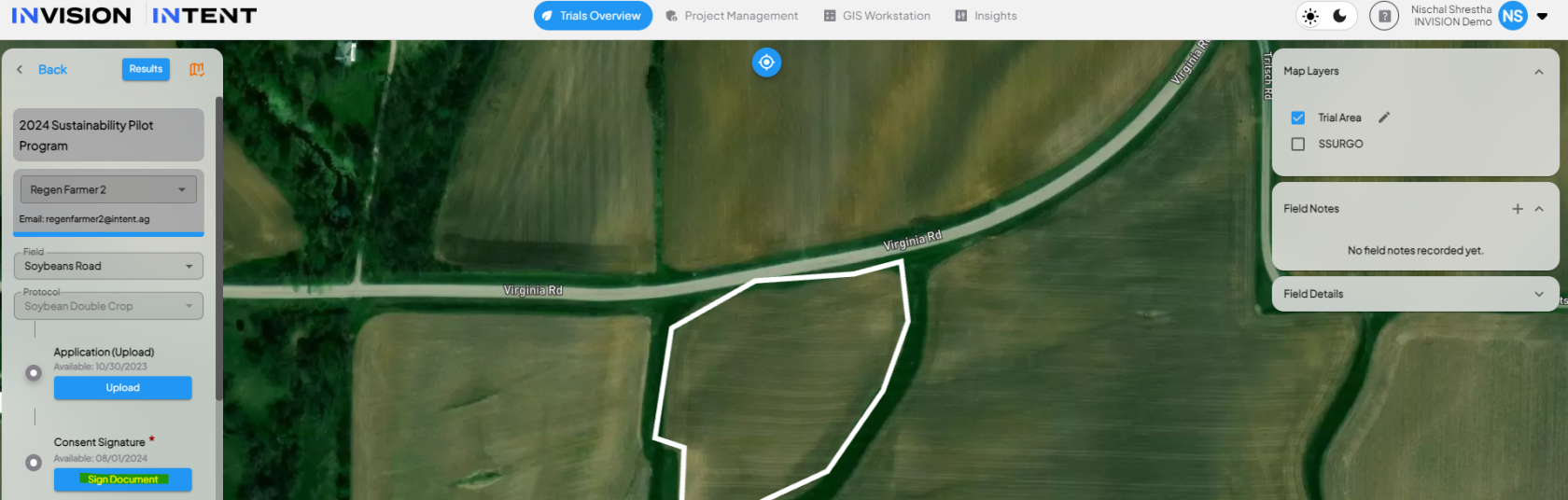
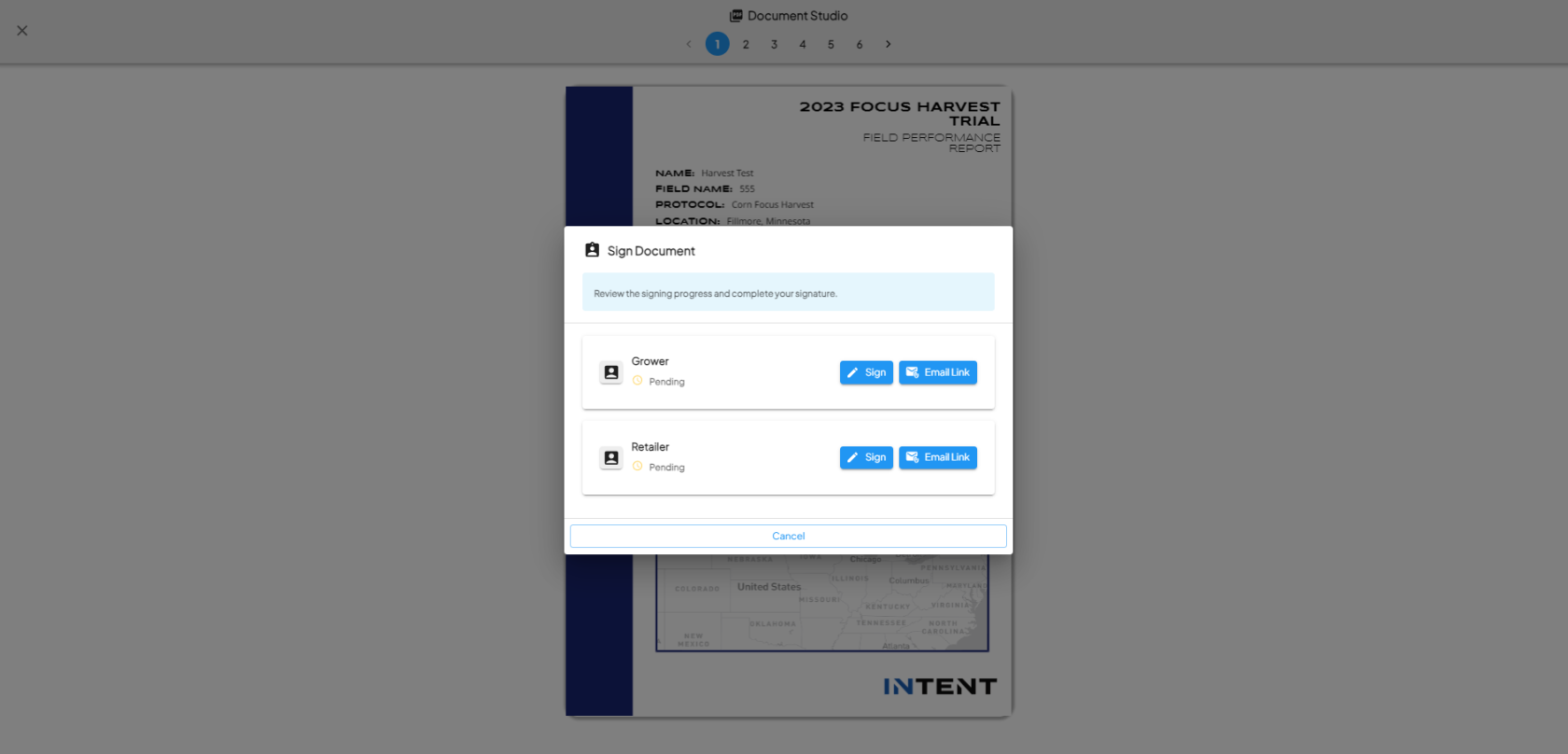
Access the 'Signature' Protocol Step
- In the View Fields section, find the 'Signature' protocol step on the left-side navigation.
-
Sign Today
-
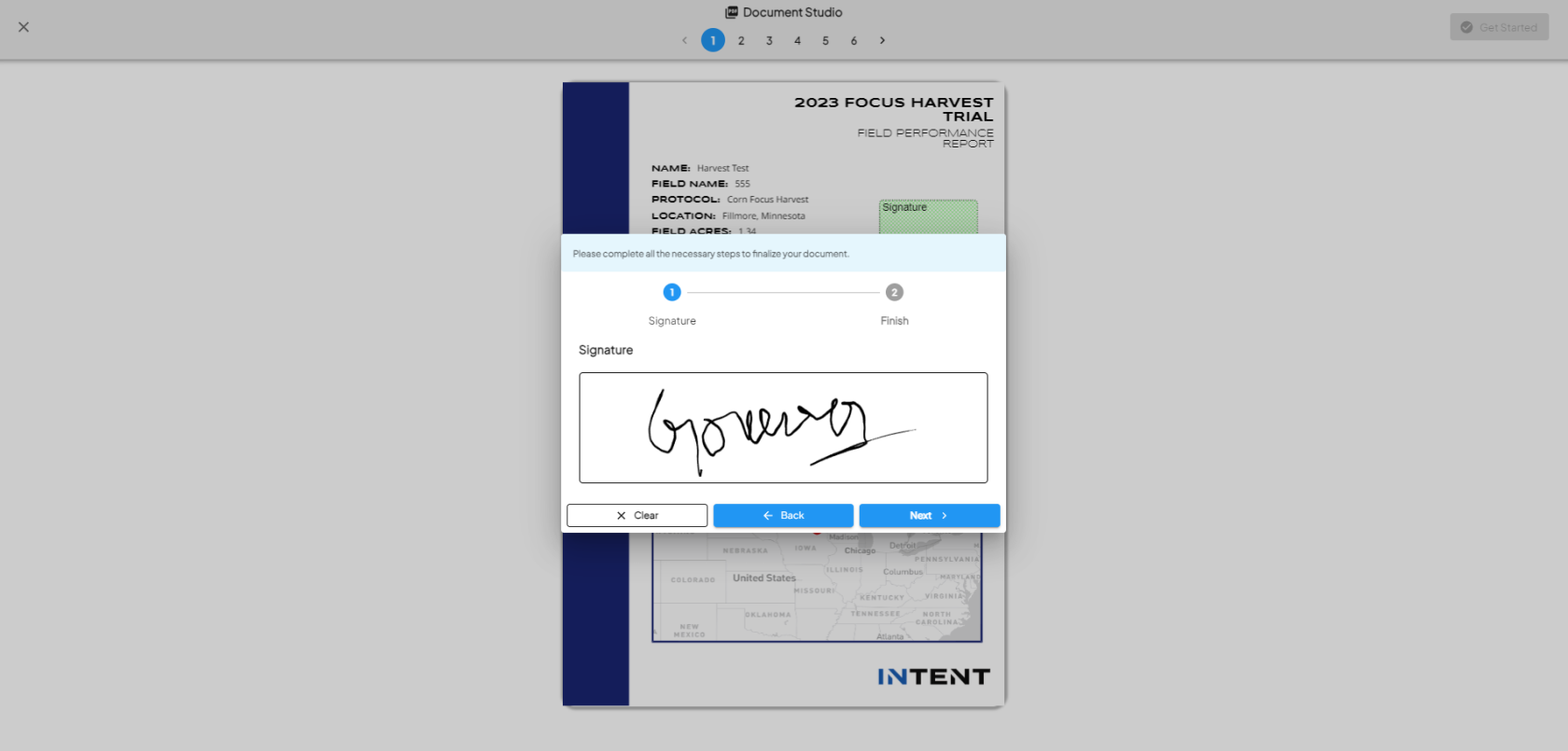
Complete the Signature Steps
- Once you pick the signer, click on Get Started.
- The PDF will display with all annotation placeholders.
- It will prompt you step by step to provide the necessary input (e.g., draw or type a signature, add text or dates).
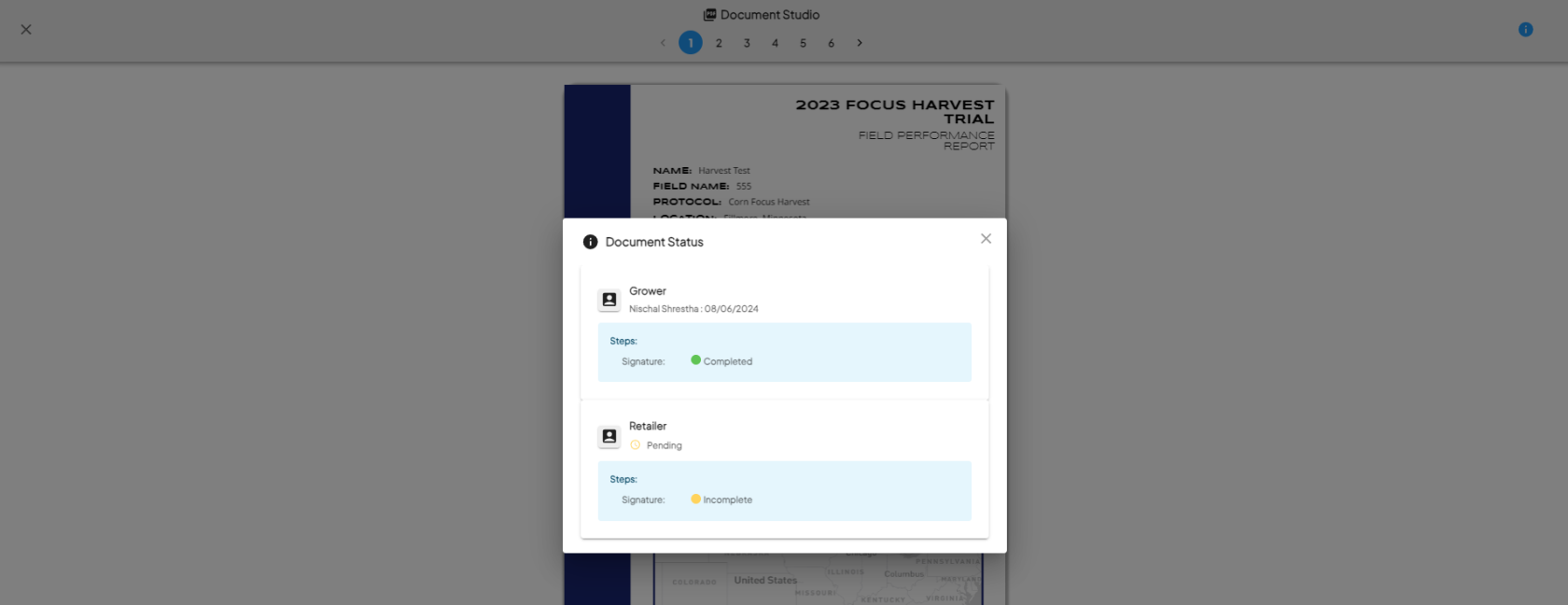
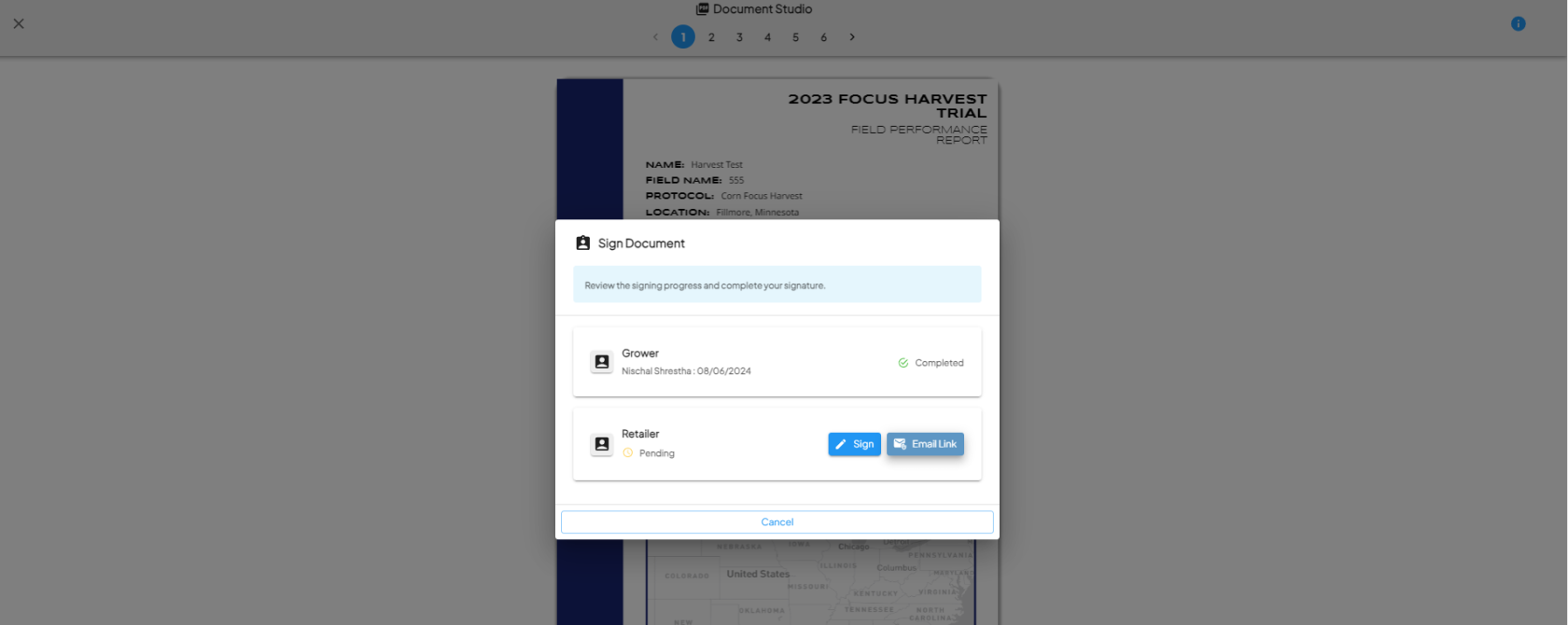
- Once all steps and signers are completed, the protocol step is marked as complete.
-
Download the Signed PDF
-
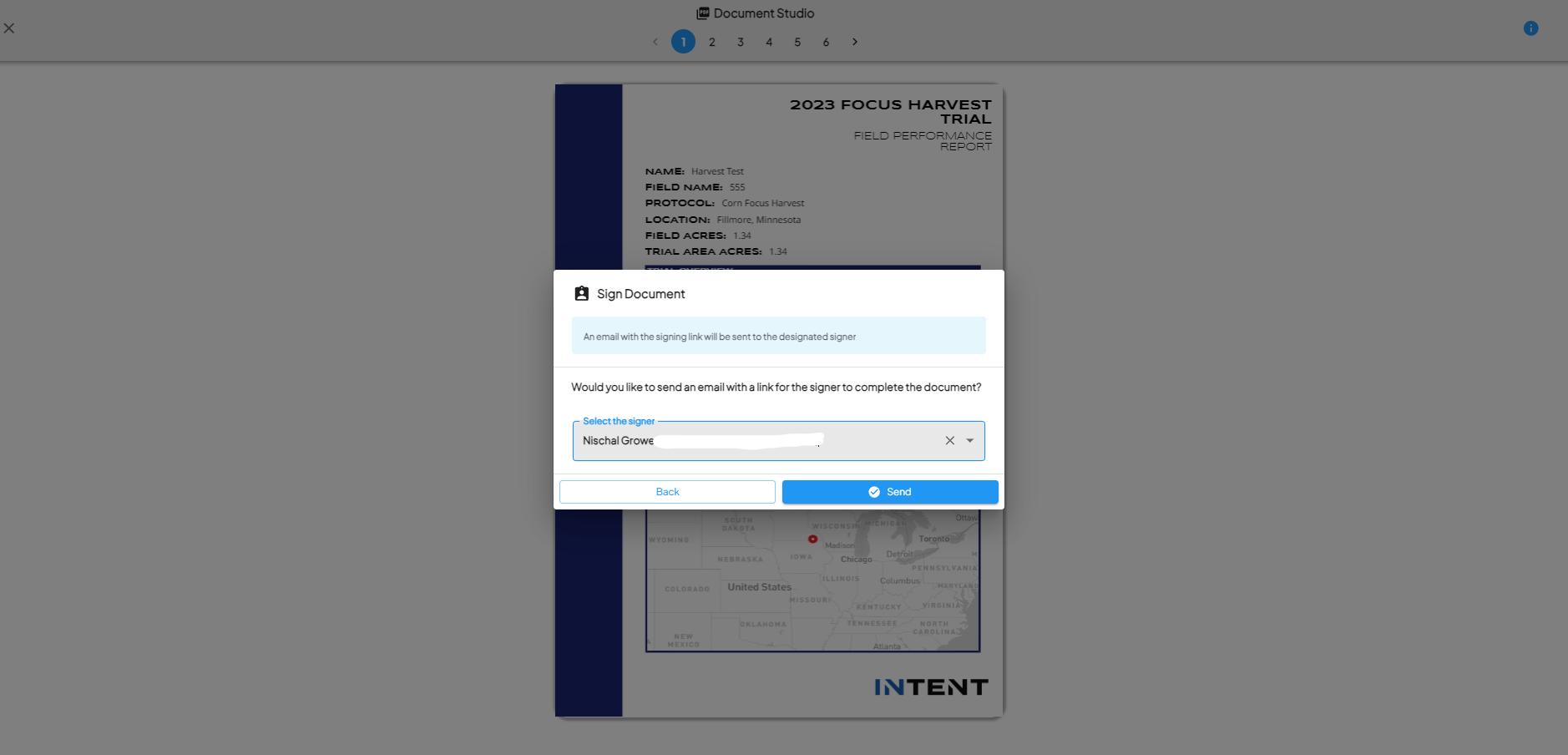
Prompt for Multiple Fields
- If you have multiple fields to sign, you will be prompted: “Would you like to sign the document signature for all the other existing fields for this grower?” Select YES or NO.
Part 3: Emailing to Request Signature Signing
-
Send Email for Signature
-
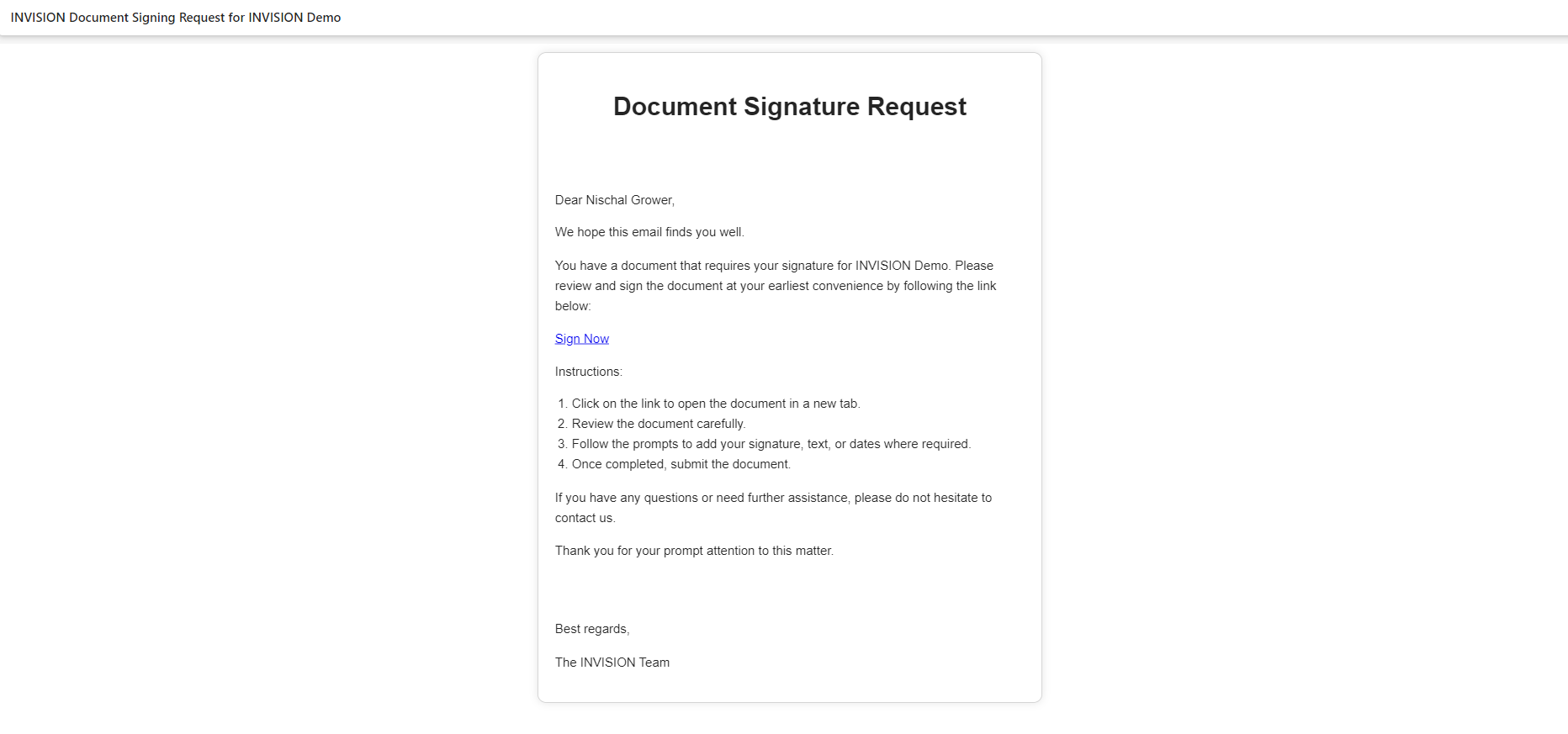
Email Sent to Grower
-
Email Content
- Signing from the email:
-
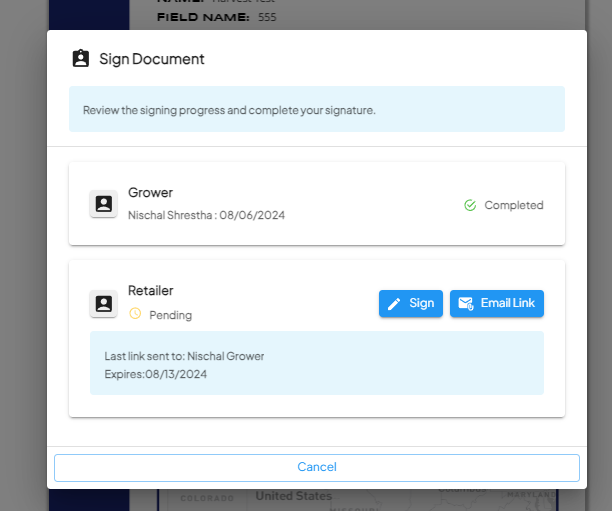
Signing Workflow
- The grower clicks the link, which opens a secure webpage to review and sign the document.
- Once signed, the system captures who signed the document, the date of completion, and any additional metadata.
-
Download Signed PDF
-
Conclusion
The Document Studio in INVISION streamlines the document signing process, making it easy for admins to set up documents, users to sign them, and request signatures via email. Follow these steps to ensure a smooth and efficient workflow for all users involved. If you have any questions or need further assistance, please contact our support team.
Example Use Cases:
-
Trial Agreements and Protocols:
- Efficiently manage and obtain signatures for trial agreements, treatment protocols, and consent forms from multiple stakeholders across various regions, reducing delays and errors.
-
Compliance and Regulatory Documents:
- Ensure all regulatory documents, such as pesticide application records, environmental impact assessments, and worker safety agreements, are properly signed and securely stored, minimizing the risk of non-compliance.
-
Grower and Land Use Contracts:
- Coordinate and track the signing of grower agreements, land use contracts, and crop management plans across different regions, ensuring all necessary documents are signed and up-to-date.
-
Field Visit and Inspection Reports:
- Streamline the process of capturing signatures for field visit reports, inspection checklists, and compliance audits conducted by agronomists and field inspectors.
-
Research and Development Documentation:
- Manage the signing of research protocols, experiment setup forms, and data sharing agreements for agricultural research and development projects.